2026 Sterilization Compliance Calendar Innovative Outstanding Superior. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial sterilization practices. Identifying whether the current or proposed alternate sterilization sites are ready for fda inspection.

Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial sterilization practices. Keep track of sterilization procedures to protect your practice & your patients with the crosstex. The environmental protection agency (epa) has set an october 5, 2026, deadline for.
 Source: www.slideshare.net
Source: www.slideshare.net
Sterilization Standards Update Strategies for Compliance PPT We are happy to share a comprehensive compliance calendar for private companies, small companies, not for profit covering secretarial, fema compliances, threshold compliances and many more! Fda recommends that affected device manufacturers who are requesting enforcement discretion submit an “informal notification” with the following information:
 Source: storage.googleapis.com
Source: storage.googleapis.com
Crosstex Sterilization Log Sheet 2023 at Lily Bloom blog Keep track of sterilization procedures to protect your practice & your patients with the crosstex. Fda recommends that affected device manufacturers who are requesting enforcement discretion submit an “informal notification” with the following information:
 Source: www.crosstex.com
Source: www.crosstex.com
SureCheck™ Sterilization Pouches (Steam) CROSSTEX Keep track of sterilization procedures to protect your practice & your patients with the crosstex. The environmental protection agency (epa) has set an october 5, 2026, deadline for.
 Source: www.crosstexbms.com
Source: www.crosstexbms.com
Tools Crosstex Biological Monitoring Service Provider Epa set 6 april 2026 as the compliance deadline for its eto final rule. Choice of immediate test failure notification by fax or email with a courtesy call the next day from a clinical advisor.
 Source: maudqmaryanne.pages.dev
Source: maudqmaryanne.pages.dev
Crosstex Sterilization Compliance Calendar Bobby Nicoli Quarterly compliance calendar 2025 for sterility assurance: Identifying whether the current or proposed alternate sterilization sites are ready for fda inspection.
 Source: www.crosstexbms.com
Source: www.crosstexbms.com
Tools Crosstex Biological Monitoring Service Provider We are happy to share a comprehensive compliance calendar for private companies, small companies, not for profit covering secretarial, fema compliances, threshold compliances and many more! As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or.
 Source: cordiqlanita.pages.dev
Source: cordiqlanita.pages.dev
2024 Sterilization Compliance Calendar Issy Melinde Identifying whether the current or proposed alternate sterilization sites are ready for fda inspection. Starting in 2026, fifra requires enhanced eto monitoring and strict compliance measures, significantly affecting commercial sterilization practices.
 Source: maudqmaryanne.pages.dev
Source: maudqmaryanne.pages.dev
Crosstex Sterilization Compliance Calendar Bobby Nicoli Keep track of sterilization procedures to protect your practice & your patients with the crosstex. Results in 24 hours for steam, 72 hours for chemical vapor and 7 days for dry heat and eto from start of incubation period at processing laboratory.
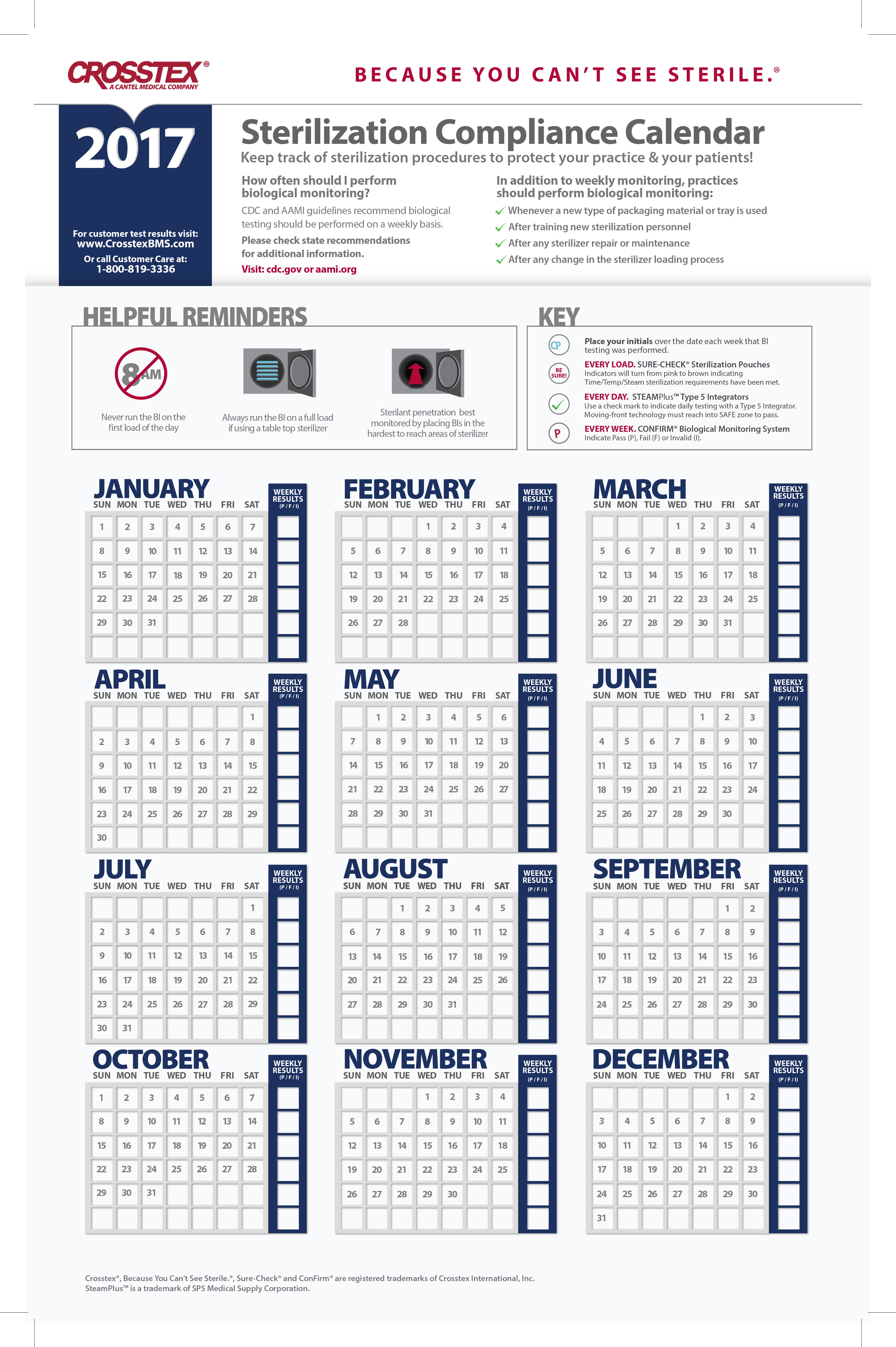 Source: www.crosstexbms.com
Source: www.crosstexbms.com
Tools Crosstex Biological Monitoring Service Provider Choice of immediate test failure notification by fax or email with a courtesy call the next day from a clinical advisor. We are happy to share a comprehensive compliance calendar for private companies, small companies, not for profit covering secretarial, fema compliances, threshold compliances and many more!
 Source: www.slideserve.com
Source: www.slideserve.com
PPT Sterilization Validation Service Market 20202026 PowerPoint Epa set 6 april 2026 as the compliance deadline for its eto final rule. Fda recommends that affected device manufacturers who are requesting enforcement discretion submit an “informal notification” with the following information:
 Source: blitheqteresa.pages.dev
Source: blitheqteresa.pages.dev
Hr Compliance Calendar 2024 Loria Raychel Results in 24 hours for steam, 72 hours for chemical vapor and 7 days for dry heat and eto from start of incubation period at processing laboratory. Epa set 6 april 2026 as the compliance deadline for its eto final rule.
 Source: bettayrenelle.pages.dev
Source: bettayrenelle.pages.dev
Sterilization Compliance Calendar 2025 Karie Leanna Quarterly compliance calendar 2025 for sterility assurance: As part of this work, fda closely monitors the supply chain effects of temporary or permanent closures and potential closures or.